Reversible Self-Assembly of N-Heterocyclic Carbene on Metal Surfaces
2022.6.10
Functionalization of metal surfaces is an important way to achieve controllable and selective adsorption, thus is of great significance for the field of gas detection and drug release. The formation of stable and tunable large-area molecular self-assembled structures (SAM) on metal surfaces is a common way to functionalize metal surfaces. N-heterocyclic carbene (NHC) molecules are easily combined with transition metal elements, forming stable self-assembled structures on metal surfaces. By adjusting the side groups, NHC SAM with specific functions can also be constructed in a targeted manner. As a kind of NHC molecule, the cyclic alkyl (amino) carbene (CAAC) molecule exhibits a stronger propensity towards σ-donation and the ability to combine with transition metal elements. Thus CAAC SAMs are more stable and provide a broader prospect in the field of surface functionalization. However, little is known about the binding modes and self-assembled structures of such molecules on metal surfaces.
Recently, Prof. Du’s group and their co-workers have investigated the adsorption configuration and the phase transition of cyCAAC SAMs on metal surfaces by combining scanning tunneling microscopy (STM) and first-principles calculations. The cyCAAC molecules were observed to form a trimer phase on Au(111) at low coverage and then transform into a dimer phase with increasing coverage (see Figure 1). Theoretical calculations revealed that the trimer phase is more stable at low coverage. As the coverage increases, the dimer phase becomes the most stable adsorption configuration. In addition, the cyCAAC molecule has two very similar spatial conformations, axial and equatorial (see Figs. 2a, b), which are indistinguishable under STM. Through theoretical calculations, the authors found that the trimer phase and the dimer phase are constructed by axial and equatorial conformations, respectively (see Figure 2). Therefore, a conformational transition from axial to equatorial happens if the trimer phase is transferred to the dimer phase. In addition, in Cu(111), cyCAAC molecules also form a long-range ordered stripe phase (see Figure 3).
This work provides an in-depth study of the binding mode, adsorption configuration, and SAMs of CAAC molecules on metal surfaces, providing a springboard for the development of advanced materials with specific functions. The related work was published in Angewandte Chemie – International Edition (https://doi.org/10.1002/anie.202115104) under the title “Reversible Self-Assembly of N-Heterocyclic Carbene on Metal Surfaces”. This research was funded by the Ministry of Science and Technology (2016YFA0202300), the National Natural Science Foundation of China (61888102), the Chinese Academy of Sciences (XDB36000000, XDB36000000, YSBR-003), and other projects.
Recently, Prof. Du’s group and their co-workers have investigated the adsorption configuration and the phase transition of cyCAAC SAMs on metal surfaces by combining scanning tunneling microscopy (STM) and first-principles calculations. The cyCAAC molecules were observed to form a trimer phase on Au(111) at low coverage and then transform into a dimer phase with increasing coverage (see Figure 1). Theoretical calculations revealed that the trimer phase is more stable at low coverage. As the coverage increases, the dimer phase becomes the most stable adsorption configuration. In addition, the cyCAAC molecule has two very similar spatial conformations, axial and equatorial (see Figs. 2a, b), which are indistinguishable under STM. Through theoretical calculations, the authors found that the trimer phase and the dimer phase are constructed by axial and equatorial conformations, respectively (see Figure 2). Therefore, a conformational transition from axial to equatorial happens if the trimer phase is transferred to the dimer phase. In addition, in Cu(111), cyCAAC molecules also form a long-range ordered stripe phase (see Figure 3).
This work provides an in-depth study of the binding mode, adsorption configuration, and SAMs of CAAC molecules on metal surfaces, providing a springboard for the development of advanced materials with specific functions. The related work was published in Angewandte Chemie – International Edition (https://doi.org/10.1002/anie.202115104) under the title “Reversible Self-Assembly of N-Heterocyclic Carbene on Metal Surfaces”. This research was funded by the Ministry of Science and Technology (2016YFA0202300), the National Natural Science Foundation of China (61888102), the Chinese Academy of Sciences (XDB36000000, XDB36000000, YSBR-003), and other projects.
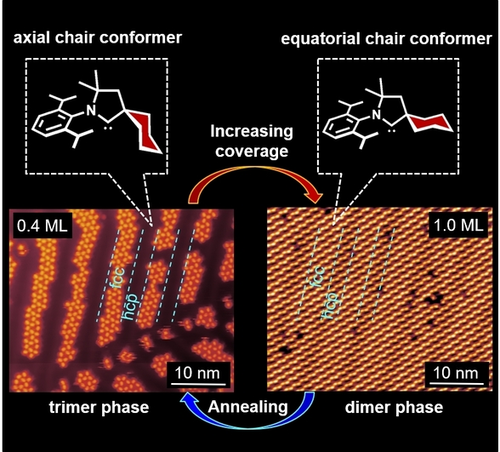
Figure 1. STM images and XPS spectrum of cyCAAC SAM on Au(111)
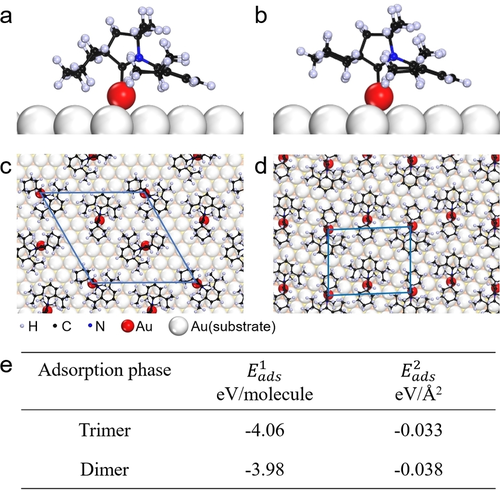
Figure 2. Adsorption configuration, atomic models and adsorption energy of cyCAAC SAM on Au(111).
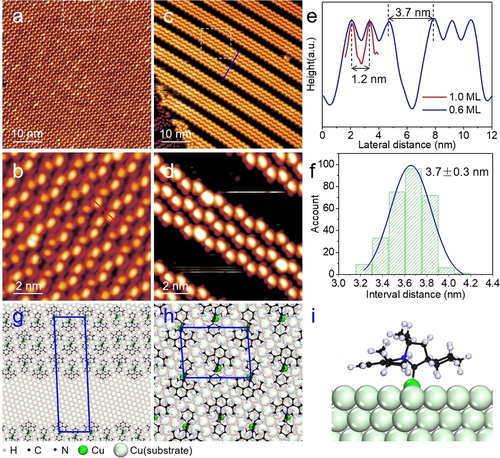
Figure 3. STM images and atomic models of cyCAAC SAM on Cu(111)
